NAD+ & NADH: what’s the difference?
There are two forms of nicotinamide adenine dinucleotide. They are chemically similar but they’re not the same thing. So, what’s the difference?
NAD+, or nicotinamide adenine dinucleotide, is a coenzyme present in every one of the body’s living cells. The “+” sign makes all the difference, despite it sometimes being dropped at the end of NAD. However, the plus sign is vital, as NAD is used to collectively refer to NAD+ and NADH – the two different NAD variations.
Still keen to learn the difference between the two molecules? Awesome, let’s find out the difference between them and how they work for your cellular health.
NAD+ & NADH: the same but different
NAD comes in two forms: NAD+ and NADH, with each playing different roles in our health and longevity. These two NAD variations are called a “redox couple”, a term used to describe a reduced (“red”) and oxidised (“ox”) form of the same molecule or atom. “Oxidised” can be misleading, however, as it does not necessarily need oxygen. Redox reactions include the loss or gain of electrons. If something becomes oxidised, it means it is losing electrons. Conversely, if something is reduced, it means it is gaining electrons
“Oxidised” has been used throughout history, and was first used in 18th century experiments. Redox reactions are not exclusive to NADH and NAD+, and are certainly not exclusive in the body. In fact, they can span everything from the formation of minerals to the rusting of iron.
Regarding NAD+, redox reactions are a necessary component of creating cellular energy. When NAD+ is converted into NADH. it gains two things: two electrons and a charged hydrogen molecule (H+). As electrons are negatively charged, the combination of H+ and NAD+, paired with the two electrons, effectively cancel each other out and neutralise the resulting NADH.
Therefore, NADH lacks the “+” sign.
A molecule’s charge informs how it works with other molecules. A molecule’s charge informs how it interacts with other molecules. For example, NADH doesn’t have the same capabilities as NAD+, and the other way around.
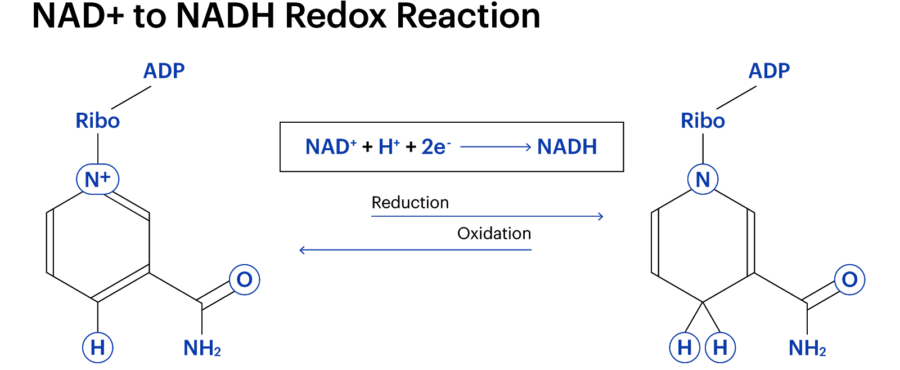
Therefore, NAD+ and NADH are very close to being the same thing, but with minor differences. However, the body doesn’t contain the same amount of each molecule. Scientists are yet to determine the NAD+ to NADH ratio, let alone what occurs when it is upset. This is vital a NAD+ has recently come to the forefront of the scientific study of longevity.
NAD+ is needed for fundamental biological processes to happen. However, the body has a limited NAD+ supply, as levels steadily decline as we age. Furthermore, NAD+ is vital to the production of sirtuins, a protein group that participates in vital cellular processes, including cellular energy production and supporting a healthy metabolism. If sirtuins are unable to access NAD+, they will not be able to function effectively.
However, some scientists believe it is the ratio of NAD+ to NADH, and not the body’s NAD+ supply, that is problematic in human ageing. This is because the ratio dictates how efficiently the cell can produce adenosine triphosphate (ATP), otherwise known as the “cell’s energy currency”.
How NAD+ & NADH work to create cellular energy
The conversion of NAD+ and NADH, and vice versa, are vital reactions for the production of ATP during “cellular respiration”. When we consume food, it goes through three phases to become the energy we use: glycolysis, the Krebs cycle and the electron transport chain.
During the glycolysis and the Krebs cycle, NADH molecules are created from NAD+, while in the electron transport chain, NAD+ is formed from splitting NADH molecules. This produces H+ and some electrons, too. The H+ are used to power something that is similar to a pump. This pump sits on the inner membrane of the mitochondria, producing lots of energy in the form of ATP. Once the H+ have gone through the pump, they eventually merge with the electrons and an oxygen molecule to create water. Each of three phases of respiration produce ATP, but the electron transport chain produces the most.
The cell uses NADH and NAD+ in other reactions not regarding ATP production, too. For example, in liver cells, the enzymes aldehyde dehydrogenase (ALDH) and alcohol dehydrogenase (ADH) use NAD+ as an oxidising agent in order to break down ethanol from alcohol beverages into a less toxic compound called acetate. In each of the enzymatic reactions, NAD+ takes an H+ from ethanol and two electrons to form NADH.
So, what is the ratio all about?
The body’s NAD+ and NADH requirements affect the ratio between the pair, which can have different effects on biological processes and cellular health. For example, drinking too much alcohol can decrease the NAD+/NADH ratio in the cytoplasm. This is due to excess conversion of NAD+ to NADH to be able to oxidise the alcohol.
NAD+ consuming enzymes, like sirtuins, also need NAD+ to be able to function effectively. As opposed to alcohol dehydrogenase and reactions in cellular respiration, these are not redox reactions, and therefore do not produce any NADH when they utilise NAD+. Instead, they use NAD+ to produce nicotinamide (a kind of vitamin B3) as a kind of byproduct.
To re-form NAD+, nicotinamide has to be recycled along what are known as “salvage pathways”. If there is a smaller amount of NAD+ available, as well as reduction in NAD+/NADH, there may be a detrimental impact on the abilities of these enzymes to function effectively. This being said, the NAD+/NADH ratio is a complex thing, and varies greatly between different cell locations.
In 1967, Krebs et al. conducted experiments on how much the ratio changed between the cytoplasm and mitochondria in rats, concluding the mitochondria ratio withheld external stressors, including starvation, where the cytoplasm ratio was greatly depleted when stressed.
There currently is no evidence to suggest that these studies can work for humans. In fact, even when the cytoplasm pool of NAD+ is greatly depleted, the mitochondrial NAD+ levels can remain strong for around three days. This is thought to be because of the inability of NAD+ to cross the mitochondrial membrane, otherwise known as the “gateway” to the mitochondria. Therefore, changes in the cytoplasmic NAD+/NADH ratio have zero effect on mitochondria. Only when a cell is in great distress, and the mitochondrial membrane is compromised, the ratio of mitochondrial NAD+/NADH is lost.
While the mitochondria’s regulation of the ratio is important, scientists have determined that these results only raise more questions about the NAD+/NADH ratio. A 2012 study about the dynamic regulation of NAD+ in the mitochondria concluded that “current knowledge leaves a number of processing questions unanswered”.
A higher ratio favours the production of more energy, however, and recently published human studies have concluded that not only does NAD+ decline as we age, but the levels of NADH in the body actually increase. This goes on to be accompanied by a steady decline in the ratio of NAD+ and NADH. Therefore, this ratio’s imbalance could affect how well the cells use NAD+.
There are ways of increasing NAD+ in the body, like supplementing NAD+ precursors. These supplements have gained scientific interest for their potential to reduce cellular decline. Human supplementation has shown to increase levels of NAD+.
Both NAD+ and NADH are vital in the production of ATP, as well as a host of other biological processes. Therefore, they are both proving to play an important role in our health. The unknowns regarding the ratio should soon be answered with further scientific study.
Related Articles
N-Acetyl-Cysteine NAC Benefits: What You Need to Know
Quercetin Supplement: What Are The Benefits?
Products
-
 NMN Nicotinamide Mononucleotide
Rated 4.90 out of 5From: $89.97 — or from
NMN Nicotinamide Mononucleotide
Rated 4.90 out of 5From: $89.97 — or fromFrom: $89.97From: $80.97 / month -
 Resveratrol
Rated 4.95 out of 5$57.97 — or
Resveratrol
Rated 4.95 out of 5$57.97 — or$57.97$52.17 / month -
 B1
Rated 5.00 out of 5From: $49.97
B1
Rated 5.00 out of 5From: $49.97 -
 Lion's Mane
Rated 5.00 out of 5$59.97
Lion's Mane
Rated 5.00 out of 5$59.97 -
 NMN + Resveratrol
Rated 4.92 out of 5
NMN + Resveratrol
Rated 4.92 out of 5From: $295.88From: $247.97 — or fromFrom: $295.88From: $223.17 / month